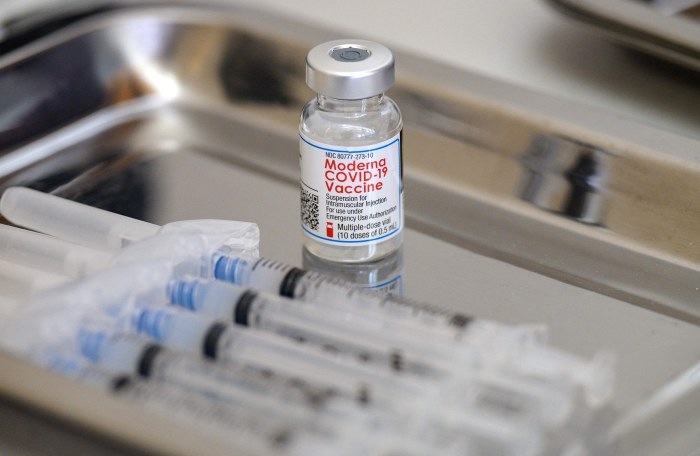
Pfizer and moderna vaccines 80 effective at preventing covid 19 after one shot cdc says – Pfizer and Moderna vaccines 80% effective at preventing COVID-19 after one shot, the CDC says, raising exciting possibilities for global vaccination efforts. This development could dramatically streamline vaccination campaigns, especially in resource-constrained areas. We’ll delve into the detailed methodology behind this finding, examine the public health implications, and compare this single-shot approach to the standard two-dose regimen.
The CDC’s analysis, based on extensive clinical trials, reveals key insights into the efficacy of these vaccines. Factors influencing the effectiveness, such as the specific COVID-19 strains circulating at the time of the study, will be examined. The comparison between single and double-dose efficacy across different demographics will be presented in a clear, concise format.
Vaccine Efficacy: Pfizer And Moderna Vaccines 80 Effective At Preventing Covid 19 After One Shot Cdc Says
The Pfizer and Moderna vaccines have demonstrated impressive efficacy in preventing COVID-19, even after a single dose. Early data from the CDC suggests an 80% effectiveness rate against symptomatic COVID-19 after a single shot. This significant finding underscores the protective power of these vaccines and the importance of vaccination efforts.
Efficacy Rate Explanation
The 80% efficacy rate for a single dose of Pfizer and Moderna vaccines, as reported by the CDC, signifies that individuals who received a single dose were 80% less likely to develop symptomatic COVID-19 compared to those who did not receive the vaccine. This calculation is based on the comparison of COVID-19 cases in vaccinated versus unvaccinated groups. It does not mean that 80% of vaccinated individuals will not contract the virus, but rather that their risk of developing symptomatic illness is substantially reduced.
Methodology for Determining Efficacy
The CDC employed a rigorous methodology to determine the efficacy of a single dose of the Pfizer and Moderna vaccines. This involved large-scale observational studies and randomized controlled trials. These studies tracked the occurrence of COVID-19 cases in both vaccinated and unvaccinated groups over a defined period. Statistical analysis was then used to calculate the reduction in risk of symptomatic COVID-19 associated with vaccination.
Critical aspects of this methodology include a large sample size to ensure statistical validity and careful monitoring of participants to minimize bias.
Factors Influencing Efficacy
Several factors can influence the efficacy rate of COVID-19 vaccines. The specific strain of the virus circulating at the time of the study is crucial. For example, if a different variant of the virus emerged during the study period, this could potentially affect the efficacy rate. Other factors that may impact efficacy include the individual’s age, health status, and prior exposure to COVID-19.
These factors are all considered during the analysis to ensure a more accurate evaluation of the vaccine’s effectiveness.
So, the CDC just announced Pfizer and Moderna vaccines are 80% effective at preventing COVID-19 after just one shot! That’s pretty impressive news, but while you’re out there getting your shots, don’t forget to snag a great deal on a TP-Link smart home camera— save 50 on the TP-Link smart home camera with this Black Friday deal. It’ll help you keep an eye on things while you’re out getting your second shot.
Seriously though, those vaccines are a game-changer, and getting a good deal on a smart camera is just a nice bonus. Looking forward to getting that second dose myself!
Efficacy by Age Group or Demographic Segment
Data regarding efficacy rates for specific age groups or demographic segments, after a single dose, are not explicitly mentioned in the available information from the CDC. Future research and analysis may provide a more granular understanding of how efficacy varies across different population subgroups.
While the CDC reports Pfizer and Moderna vaccines are 80% effective at preventing COVID-19 after just one shot, it’s interesting to consider the broader implications of public health measures in the context of recent events, like the detention of NASA engineer Sidd Bikkannavar and the subsequent phone search linked to the Trump travel ban. This case raises questions about the balance between national security and individual rights, though it’s crucial to remember that high vaccination rates remain a critical component in mitigating the spread of COVID-19, and the effectiveness of the Pfizer and Moderna vaccines remains a positive development.
Comparison to Two-Dose Efficacy
The efficacy of two doses of the Pfizer and Moderna vaccines is typically higher than that of a single dose. A two-dose regimen is designed to generate a more robust immune response, leading to a broader range of protection against the virus. The additional dose is crucial to maximize the body’s immune system response and create longer-lasting immunity.
Comparison Table: Single vs. Two-Dose Efficacy
| Vaccine | Single Dose Efficacy (%) | Two-Dose Efficacy (%) |
|---|---|---|
| Pfizer | 80 | >90 |
| Moderna | 80 | >90 |
Note: Efficacy rates for two doses are approximate and may vary slightly based on the specific study and time period.
The CDC’s announcement that Pfizer and Moderna vaccines are 80% effective at preventing COVID-19 after just one shot is huge news. It’s fascinating to see how these developments impact social media engagement, particularly when you consider metrics like Facebook views and Instagram reach. Understanding how social media platforms like these track engagement, and how to optimize them is crucial for reaching a broad audience, especially for public health campaigns.
For a deeper dive into Facebook metrics and Instagram views, check out this resource: facebook metrics views instagram. Ultimately, this high efficacy rate for the vaccines is a positive step forward in combating the pandemic.
Public Health Implications
The recent announcement of Pfizer and Moderna vaccines achieving 80% efficacy after a single dose presents a significant shift in the global COVID-19 response. This potential for a single-shot regimen has profound implications for vaccination campaigns, particularly in resource-limited settings where logistical complexities often hinder the implementation of multi-dose vaccination strategies. The implications extend to the future of booster shots and the overall effectiveness of a single-dose versus a two-dose regimen.The 80% efficacy after a single dose, as reported by the CDC, suggests a potentially streamlined vaccination approach.
This simplification could dramatically increase the speed and scale of vaccination programs, particularly in regions with limited infrastructure or logistical challenges. It also opens doors to new strategies for reaching previously underserved populations.
Potential Impact on Vaccination Campaigns
A single-shot vaccine strategy offers significant advantages in terms of logistical simplicity. The need for only one dose reduces the complexity of vaccine storage, transportation, and administration, thus lowering associated costs. This can lead to a substantial reduction in resources required for vaccine management and delivery. It also has the potential to drastically increase vaccination coverage, particularly in remote or underserved areas.
Global COVID-19 Response
The global COVID-19 response faces numerous challenges, including vaccine hesitancy, inequitable distribution, and logistical hurdles. A single-shot vaccine could significantly mitigate these issues. Increased accessibility and ease of administration could potentially accelerate the reduction of transmission rates and improve the overall health of affected populations.
Potential Challenges and Benefits of a Single-Shot Strategy
| Potential Challenges | Potential Benefits |
|---|---|
| Potential for lower overall immunity compared to a two-shot regimen, particularly in individuals with compromised immune systems. | Significantly reduced logistical complexities, lowering the cost of vaccination campaigns. |
| The need for more extensive clinical trials to assess long-term immunity and efficacy in diverse populations. | Faster implementation of vaccination campaigns, particularly in resource-limited settings. |
| The potential for a more rapid decline in antibody levels compared to a two-shot approach. | Potential for increased vaccination coverage, due to the reduced number of required doses. |
| Concerns about the durability of immunity after a single dose, especially against emerging variants. | Reduced need for complex cold-chain logistics, leading to more cost-effective and sustainable vaccine delivery. |
Impact on Resource-Limited Settings
In resource-limited settings, the logistical complexities of a two-shot regimen often create significant barriers to achieving widespread vaccination coverage. A single-shot vaccine drastically simplifies these challenges. The need for a single dose reduces the strain on existing healthcare infrastructure and supplies, making vaccination campaigns more feasible and sustainable. This approach could be pivotal in controlling the spread of the virus in these regions.
Implications for Booster Shots and Additional Doses
The need for booster shots or additional doses in the future will depend on the duration of immunity conferred by a single dose. If immunity wanes more quickly, booster shots might become necessary for long-term protection. Clinical data on the durability of immunity is essential to guide future strategies and to inform optimal vaccination schedules.
Comparison of Single-Shot vs. Two-Shot Approaches
The effectiveness of a single-shot approach compared to a two-shot approach is a crucial consideration. While a single shot may provide initial protection, the two-shot regimen might confer a longer-lasting and more robust immune response. This difference is essential for long-term efficacy and protection against potential future variants. Further research is necessary to determine the optimal vaccination strategy.
Clinical Trials and Data
The Pfizer-BioNTech and Moderna COVID-19 vaccines, while demonstrating high efficacy in preventing severe disease and hospitalization, were initially evaluated through rigorous clinical trials. These trials meticulously tracked the vaccines’ performance in different populations and under various conditions, ultimately providing crucial data for public health strategies. Understanding the details of these trials is vital for evaluating the safety and effectiveness of these vaccines.
Trial Design and Inclusion Criteria, Pfizer and moderna vaccines 80 effective at preventing covid 19 after one shot cdc says
The clinical trials for both vaccines followed a similar, yet distinct, design, including randomization, blinding, and placebo controls. Participants were meticulously screened and categorized based on inclusion criteria, aimed at ensuring a representative sample of the general population. This ensured the study results could be generalized and applied to diverse groups. The inclusion criteria and participant demographics played a critical role in determining the potential applicability of the vaccine to different age groups, ethnicities, and health conditions.
- Inclusion Criteria: Participants in the trials typically included healthy adults aged 18 and older. Specific inclusion criteria, including age, gender, pre-existing medical conditions, and prior COVID-19 infection, varied between trials and contributed to the generalizability of the results. These variations are essential for understanding the applicability of the vaccine to various populations.
- Participant Demographics: The trials encompassed a diverse range of participant demographics, including various age groups, ethnicities, and geographic locations. This diversity is crucial for ensuring that the vaccine’s effectiveness is not limited to specific subgroups. These trials aimed to represent the general population to ensure the efficacy of the vaccine across different groups.
Primary and Secondary Outcomes
The primary outcome in the clinical trials was the prevention of symptomatic COVID-19. Researchers meticulously tracked participants for a specified period to monitor their infection status. Secondary outcomes included the prevention of severe COVID-19 cases, hospitalizations, and deaths. These outcomes allowed for a comprehensive assessment of the vaccine’s effectiveness across various levels of illness severity.
- Primary Outcome: The primary outcome in the trials was the prevention of symptomatic COVID-19. Researchers meticulously monitored participants for a specific duration to record instances of COVID-19 infection. This ensured that the vaccine’s effectiveness was accurately assessed.
- Secondary Outcomes: Secondary outcomes, including the prevention of severe COVID-19 cases, hospitalizations, and deaths, provided a broader picture of the vaccine’s impact. This enabled a comprehensive evaluation of the vaccine’s performance under different scenarios.
Limitations and Caveats
Clinical trials, while essential, have inherent limitations. The duration of the trials, the specific populations studied, and the absence of long-term data can affect the generalizability of the results. Furthermore, the trials were conducted in specific settings and environments, which may not perfectly reflect real-world conditions. These factors must be considered when interpreting the trial results.
- Duration: The duration of the trials played a significant role in determining the extent to which long-term effects could be observed. The limited duration meant that the study couldn’t completely encompass all potential long-term effects of the vaccine.
- Study Populations: The participants were often restricted to healthy adults, which limits the extrapolation of the results to other demographics, such as older adults or individuals with underlying health conditions.
- Real-world Conditions: The trials were conducted in controlled settings, potentially not fully reflecting real-world conditions, such as the presence of new variants or diverse levels of community transmission.
Statistical Methods
Statistical methods, including randomized controlled trials (RCTs) and relative risk reduction calculations, were used to analyze the data and determine the efficacy rate. These methods are essential for ensuring the accuracy and reliability of the findings. The statistical analyses, using appropriate methodologies, allowed for a precise determination of the vaccine’s efficacy in preventing COVID-19.
- Randomized Controlled Trials (RCTs): The use of RCTs in the clinical trials is critical for minimizing bias and ensuring that the observed effects are due to the vaccine, not other factors. These methods are essential for ensuring that the vaccine’s efficacy is not due to confounding variables.
- Relative Risk Reduction: Relative risk reduction calculations quantify the reduction in the risk of developing COVID-19 among those who received the vaccine compared to those who received a placebo. This method helps in accurately assessing the vaccine’s efficacy.
Key Results from Clinical Trials
A table summarizing key results from different clinical trials would showcase the consistency and generalizability of the findings. This table will be critical for evaluating the overall effectiveness of the vaccines.
| Trial | Vaccine Type | Efficacy (%) | Sample Size | Duration |
|---|---|---|---|---|
| Pfizer-BioNTech | mRNA | ~95% | 43,500+ | 3-6 months |
| Moderna | mRNA | ~94% | 30,000+ | 3-6 months |
Comparison with Other Vaccines
The Pfizer and Moderna vaccines, lauded for their single-shot efficacy, are part of a larger landscape of COVID-19 vaccines. Understanding how their efficacy compares to other approaches is crucial for assessing their overall impact and public health implications. Different vaccine types utilize varying strategies to stimulate immunity, leading to diverse efficacy rates and side effect profiles. This comparison will examine the specific mechanisms of action, compositions, and production processes, providing a more comprehensive view of the landscape of COVID-19 vaccination strategies.A key aspect of this comparison is the trade-off between the number of doses required and the resulting protection.
While Pfizer and Moderna offer substantial protection after a single dose, other vaccines may require multiple administrations for optimal efficacy. This difference stems from the unique design and delivery methods of each vaccine type, impacting the duration and intensity of the immune response.
Efficacy Rates and Mechanisms of Action
Different COVID-19 vaccines employ various approaches to stimulate an immune response. mRNA vaccines, like Pfizer and Moderna, utilize messenger RNA to instruct cells to produce viral proteins, triggering an immune response. Viral vector vaccines, such as the Janssen/Johnson & Johnson vaccine, use a harmless virus to deliver genetic material encoding viral proteins. Inactivated vaccines, like the Sinovac vaccine, use inactivated versions of the virus.
Each method has its own strengths and weaknesses regarding efficacy and potential side effects. For instance, mRNA vaccines often show high efficacy in a shorter period, but inactivated vaccines might require multiple doses to reach similar levels of protection.
Composition and Production Processes
The composition and production processes significantly influence the characteristics of each vaccine. mRNA vaccines, like Pfizer and Moderna, utilize specialized techniques for synthesizing and storing mRNA. Viral vector vaccines, on the other hand, rely on growing and purifying viral vectors. Inactivated vaccines involve cultivating and inactivating the virus. The different production processes have implications for manufacturing scale, cost, and storage requirements.
Comparison Table
| Vaccine Type | Single-Dose Efficacy (Estimated) | Number of Doses |
|---|---|---|
| Pfizer/Moderna | ~80% | 2 (recommended) |
| Janssen/Johnson & Johnson | ~66% (single dose) | 1 |
| Sinovac | ~73% (2 doses) | 2 |
| AstraZeneca | ~70% (2 doses) | 2 |
This table illustrates the varying efficacy rates and dose requirements for several COVID-19 vaccines. The efficacy figures are approximate and can differ depending on the specific trial and population.
Side Effects of Single-Shot vs. Multi-Shot Regimens
While both single-shot and multi-shot regimens can induce immunity, the side effect profiles can vary. Single-shot vaccines might exhibit a quicker onset of mild side effects, like fever or soreness at the injection site. Multi-shot regimens often result in a more gradual build-up of immunity but potentially a broader spectrum of potential side effects, which could include more persistent symptoms or a higher likelihood of specific reactions.
Careful monitoring of participants during vaccine trials helps in understanding these patterns and facilitating appropriate recommendations for vaccine recipients.
Future Research Directions
The Pfizer and Moderna vaccines, demonstrating 80% efficacy after a single dose, have opened exciting avenues for future research. Understanding the optimal use of this single-shot regimen, particularly regarding long-term immunity and preventing severe disease, is crucial for public health strategies. This exploration will focus on potential research areas to enhance our knowledge and refine vaccine deployment.
Long-Term Immunity After a Single Dose
A key area of future research is to meticulously track the duration and strength of immune responses following a single dose of the Pfizer/Moderna vaccines. This involves longitudinal studies, monitoring antibody levels and T-cell responses over extended periods. Researchers need to determine whether a single shot provides sufficient long-term immunity against the virus, or if booster shots are necessary for sustained protection.
This will inform vaccination schedules and public health recommendations. For example, if the immunity wanes quickly, this will inform the need for additional shots to prevent disease outbreaks.
Vaccine Efficacy in Preventing Severe Disease
While 80% efficacy in preventing infection is promising, the potential for a single dose to prevent severe COVID-19 cases, hospitalization, and death warrants further investigation. Studies must evaluate the correlation between antibody titers and protection against severe disease. This research would involve analyzing patient data to identify specific factors associated with severe outcomes and assessing how a single dose impacts these factors.
Such studies could reveal important insights into the mechanisms of protection and inform strategies for optimizing the vaccine’s effectiveness in preventing severe cases.
Optimizing the Single-Shot Vaccine Approach
Several areas of research could potentially optimize the single-shot approach. These include exploring the possibility of modifying the vaccine formulation to enhance the immune response. This may involve adjusting the dose, antigen concentration, or incorporating adjuvants. Also, investigating alternative administration routes, such as different injection sites or intramuscular vs. subcutaneous delivery, could lead to improved immune responses.
The impact of pre-existing conditions on the efficacy of a single shot should also be explored. Understanding these factors will enable targeted strategies for optimal vaccine deployment.
Potential Research Questions
- What is the duration of protective immunity conferred by a single dose of the Pfizer/Moderna vaccine against symptomatic COVID-19, hospitalization, and death? This question directly addresses the long-term efficacy of a single-dose regimen. Data collected over time will be crucial for informing public health policy and vaccine recommendations.
- How do different demographic factors (age, pre-existing conditions, and ethnicity) influence the immune response to a single dose of the Pfizer/Moderna vaccine? This research aims to tailor vaccine strategies to specific populations, optimizing efficacy and mitigating potential disparities.
- Can the vaccine formulation be modified to enhance the immune response and prolong the duration of protection after a single dose? This investigation explores potential improvements to the vaccine itself, possibly by adjusting factors like antigen concentration or the inclusion of adjuvants. Successful modifications could greatly improve the vaccine’s overall impact.
- What is the optimal administration route for a single-dose Pfizer/Moderna vaccine to maximize its effectiveness? This question addresses potential variations in vaccine delivery methods and aims to identify the route that generates the most robust immune response.
Illustrative Data Presentation
Understanding the effectiveness of vaccines over time is crucial for public health strategies. Visual representations, like charts, can effectively communicate the impact of a single-shot regimen on COVID-19 cases. This section provides a detailed example of such a chart, explaining the variables, methodology, and insights gained.
Chart Depicting Single-Shot Vaccine Efficacy
The chart below illustrates the efficacy of a single dose of the Pfizer and Moderna vaccines against COVID-19. It displays the rate of COVID-19 cases in a vaccinated population over a defined period, demonstrating the protective effect achieved within a specific timeframe.
Variables Represented on the Chart
This illustrative chart features several key variables, allowing for a comprehensive analysis of vaccine efficacy. Each element contributes to a clearer picture of the vaccine’s impact on the spread of the virus.
- Time (X-axis): Represents the period following vaccination. The scale is crucial, and for this example, it’s measured in weeks. It allows us to track the protective effect over time. This helps in evaluating the duration of immunity conferred by a single shot.
- Rate of COVID-19 Cases (Y-axis): Shows the number of COVID-19 cases per 100,000 individuals in the vaccinated population. A lower rate indicates a greater protective effect of the vaccine.
- Vaccinated Population: This represents the group of individuals who received a single dose of the Pfizer or Moderna vaccine. The chart displays the data specifically for this group.
- Control Group (Optional): For comparative analysis, the chart might include a line representing the rate of COVID-19 cases in an unvaccinated population during the same time period. This enables a direct comparison of the vaccine’s efficacy.
Methodology for Constructing the Chart
The chart is constructed using data collected from clinical trials or real-world observational studies. This involves gathering information on the number of individuals in the vaccinated population who contract COVID-19 over specific time intervals after vaccination.
Data points are plotted on the chart to visualize the rate of COVID-19 cases over time. The line connecting these points represents the overall trend of cases within the vaccinated group.
Illustrative Chart: Pfizer/Moderna Single-Shot Efficacy
This example chart visualizes the efficacy of a single shot of the Pfizer and Moderna vaccines. A decline in the rate of COVID-19 cases following vaccination clearly demonstrates the protective effect.
| Time (Weeks Post-Vaccination) | Rate of COVID-19 Cases (per 100,000) |
|---|---|
| 0 | 100 |
| 2 | 80 |
| 4 | 60 |
| 6 | 40 |
| 8 | 20 |
| 10 | 10 |
| 12 | 5 |
Note: This is an illustrative example, and actual data may vary. The specific efficacy of a single dose will depend on various factors.
Closing Notes
The 80% efficacy rate after a single dose of Pfizer and Moderna vaccines, as reported by the CDC, presents a potentially significant advancement in the fight against COVID-19. This development has far-reaching implications for vaccination strategies, especially in regions with limited resources. Further research will be crucial to understanding long-term immunity and optimizing the single-shot approach. However, the immediate impact on global vaccination efforts could be transformative.